Opis
ZAPOBIEGANIE KOROZJI – STANOWISKO SZKOLENIOWE OCHRONY KATODOWEJ
Ochrona katodowa to technika kontrolowania korozji powierzchni metalu poprzez uczynienie jej katodą ogniwa elektrochemicznego. Osiąga się to poprzez umieszczenie w kontakcie z chronionym metalem innego, łatwiej ulegającego korozji metalu, który działa jako anoda ogniwa elektrochemicznego. Systemy ochrony katodowej są najczęściej stosowane do ochrony stalowych, wodnych lub paliwowych rurociągów i zbiorników magazynowych, stalowych pali, statków, morskich platform wiertniczych i lądowych obudów szybów naftowych.
Stanowisko powinno umożliwiać badanie systemów izolowanych, jak również systemów, w których różne metale są ze sobą połączone. Szczególną uwagę należy zwrócić na obecność lub brak kilku rodzajów materiałów izolacyjnych na powierzchniach próbek, aby zademonstrować różne zachowanie tego samego materiału, gdy jest pokryty lub nie.
Stanowisko powinno zapewniać odpowiednie urządzenia do podkreślenia koncepcji swobodnego potencjału korozyjnego, mierzonego za pomocą łatwych w użyciu elektrod odniesienia i środków odpowiednich do tworzenia z pewną dokładnością krzywych polaryzacji.
Techniki ochronne powinny być reprezentowane jako systemy anod protektorowych dla kilku rodzajów metali, jak również jako systemy ochrony katodowej prądem udarowym z możliwością sprawdzenia, jakie jest wyjaśnienie zastosowania stałego napięcia, stałego prądu i stałego potencjału.
Stanowisko powinno być wyposażone w urządzenia pomiarowe charakteryzujące się odpowiednią czułością i dokładnością, w celu wprowadzenia, które muszą być podstawą testów laboratoryjnych do wykonania, aby rozpoznać, który jest właściwy sposób w celu określenia zachowania metalu w kontakcie z elektrolitem w różnych warunkach temperatury (kąpiel termostatyczna) i w wysokim stężeniu tlenu (pompa wdmuchująca powietrze).
Odpowiedni interfejs wielokanałowy powinien umożliwiać podłączenie stanowiska do komputera PC w celu rejestrowania wyników eksperymentów i przesyłania śladów do dalszych badań.
Lista eksperymentów:
1) Użycie woltomierza
Najważniejszym przyrządem w dziedzinie ochrony katodowej jest woltomierz; zazwyczaj najczęściej spotykany jest typ cyfrowy, ponieważ jego duża impedancja umożliwia pomiar napięć (potencjałów) ze źródeł o bardzo wysokiej impedancji wewnętrznej.
Pomiary powinny być zgodne z wprowadzeniem do pomiarów elektrycznych i wprowadzeniem do prawa Ohma, które reguluje przepływ prądu do pierwszego i drugiego rodzaju przewodników (metali i elektrolitów).
2) Pomiar różnicy potencjałów próbki w elektrolicie.
Ten eksperyment wprowadzi do tematu ochrony katodowej. Celem tej dyscypliny będzie modyfikacja potencjału (w stosunku do ogniwa odniesienia) struktury, która ma być chroniona poprzez spowolnienie naturalnej tendencji metalu do przechodzenia do roztworu.
Eksperyment ten powinien podkreślać elektrochemiczne podejście do zjawiska korozji.
3) Ogniwo odniesienia
Eksperyment ten przedstawi praktyczne zastosowanie trzech typów ogniw wzorcowych najczęściej stosowanych w tej dyscyplinie, czyli ogniwa wzorcowego Cu/CuSO4, ogniwa wzorcowego Ag/AgCl i ogniwa wzorcowego cynkowego.
4) Ogniwo Daniela
W ogniwie Daniela elektrody miedziana i cynkowa są zanurzone odpowiednio w roztworze siarczanu miedzi (II) i siarczanu cynku. Na anodzie cynk zostanie utleniony w następującej reakcji połówkowej: Zn(s) Zn2+(aq) + 2e-
Na katodzie miedź zostanie zredukowana zgodnie z następującą reakcją: Cu2+(aq) + 2e- Cu(s)
W ogniwie Daniela, które ze względu na swoją prostotę jest często używane do demonstracji, elektrony „wyciągane” z cynku wędrują przez drut, dostarczając prąd elektryczny, który oświetla żarówkę. W takim ogniwie ważną rolę odgrywają jony siarczanowe. Posiadając ładunek ujemny, aniony te gromadzą się wokół anody, aby utrzymać ładunek neutralny.
I odwrotnie, na katodzie gromadzą się kationy miedzi (II), aby utrzymać ten neutralny ładunek. Te dwa procesy spowodują gromadzenie się miedzi na katodzie i „rozpuszczenie” elektrody cynkowej w roztworze.
5) Przewodniki pierwszego i drugiego rodzaju
Za pomocą prostego obwodu możliwe będzie wykazanie równoważności między elektrolitami i zwykłymi przewodnikami, jeśli chodzi o przepływ prądu elektrycznego.
6) Wprowadzenie do kryteriów ochrony katodowej
Za pomocą ogniwa elektrolitycznego stanowiska możliwe będzie odtworzenie zastosowania kryteriów NACE, które potwierdzają stan ochrony katodowej konstrukcji.
7) Wprowadzenie do anod protektorowych Zn, Mg i Al
Korzystając z ogniwa elektrolitycznego na stanowisku, możliwe będzie odtworzenie zastosowania anody protektorowej do konstrukcji stalowej i jednoczesne porównanie dwóch próbek, jednej w trybie ochrony katodowej, drugiej w trybie wolnej korozji.
8) Wprowadzenie do systemu prądu udarowego ochrony katodowej
Dzięki zastosowaniu ogniwa elektrolitycznego na stanowisku możliwe będzie odtworzenie zastosowania prądu udarowego do konstrukcji stalowej i jednoczesne porównanie dwóch próbek, jednej w reżimie ochrony katodowej, uzyskanej za pomocą anod protektorowych, drugiej napędzanej systemem prądu udarowego. W tym eksperymencie zostanie podkreślony wzrost zakłóceń spowodowanych obecnością systemu prądu udarowego z powodu niewłaściwego rozmieszczenia dwóch systemów.
9) Zużywalna anoda prądowa (Fe)
Dzięki zastosowaniu ogniwa elektrolitycznego na stanowisku możliwe będzie odtworzenie zastosowania prądu udarowego do konstrukcji stalowej i zaobserwowanie w czasie efektu zużycia anody z powodu jej przejścia do roztworu.
10) Anoda obojętna (Ti-Pt i MMO)
Nie wszystkie materiały anodowe przechodzą przez roztwór, dwa przykłady zostaną pokazane przy użyciu anody platynowanej tytanem i anody tytanowej pokrytej tlenkiem metalu.
11) Koncepcja rezystancji, obwód dla przewodników pierwszego i drugiego gatunku
Dzięki zastosowaniu ogniwa elektrolitycznego na stanowisku możliwe będzie wytworzenie przepływu prądu do kąpieli i w ten sposób zademonstrowanie ważności prawa Ohma w dziedzinie ochrony katodowej.
Prawo Ohma ma zastosowanie do obwodów elektrycznych; stwierdza, że prąd płynący przez przewodnik między dwoma punktami jest wprost proporcjonalny do różnicy potencjałów (tj. spadku napięcia lub napięcia między dwoma punktami) i odwrotnie proporcjonalny do rezystancji między nimi.
Równanie matematyczne opisujące tę zależność to: I = V/R
Gdzie I to prąd w amperach, V to różnica potencjałów w woltach, a R to parametr obwodu zwany rezystancją (mierzony w omach, również równoważny woltom na amper). Różnica potencjałów jest również znana jako spadek napięcia i czasami jest oznaczana jako U, E lub emf (siła elektromotoryczna) zamiast V.
12) Wprowadzenie do koncepcji oporu właściwego dla trzech różnych przewodników pierwszego rodzaju (Fe; Cu; Fe-Ni). Aby zapoznać uczniów z pojęciem rezystywności, przeprowadzony zostanie eksperyment z wykorzystaniem trzech geometrycznie identycznych próbek różnych materiałów w celu zidentyfikowania pojęcia rezystancji właściwej, która „in fieri” jest rezystywnością lub odwrotnością koncepcji przewodnictwa.
13) Wprowadzenie do koncepcji interferencji spowodowanej obecnością zewnętrznych pól elektrycznych na zakopanych lub zanurzonych strukturach (prądy błądzące). Eksperyment powinien odtwarzać wpływ zewnętrznego pola elektrycznego na zanurzoną strukturę, w wyniku czego na powierzchni próbki powstają oddzielne obszary anodowe i katodowe. Będzie to wprowadzenie do koncepcji interferencji spowodowanej obecnością zewnętrznego i zakłócającego pola elektrycznego na zakopanych lub zanurzonych strukturach (prądy błądzące).
14) Wpływ obecności powietrza na rezystywność (efekt insufflate air) – Eksperyment ten wyjaśni i zademonstruje zmianę rezystywności wraz ze wzrostem obecności powietrza rozpuszczonego w elektrolicie.
15) Wprowadzenie gęstości prądu i konstrukcja krzywych Tafela – Koncepcja gęstości prądu jest, podobnie jak różnica potencjałów, główną koncepcją w dziedzinie ochrony katodowej, a ten eksperyment pozwoli zrozumieć, że dzięki tej koncepcji możliwe będzie przewidzenie ilości prądu potrzebnego do uzyskania reżimu ochrony katodowej na znanej strukturze powierzchni zanurzonej w elektrolicie. Korzystając z dostarczonego interfejsu wielokanałowego, możliwe będzie rejestrowanie zmian wartości prądu w czasie, a następnie tworzenie krzywych polaryzacji na wykresie.
16) Wpływ temperatury na gęstość prądu (ogniwo termostatyczne) – Eksperyment ten wyjaśnia i demonstruje zmianę gęstości prądu w funkcji temperatury oraz wprowadza pojęcie aktywności chemicznej.
17) Wpływ obecności powietrza na gęstość prądu (efekt wdmuchiwanego powietrza) – Eksperyment ten wyjaśni i zademonstruje zmianę gęstości prądu w funkcji wzrostu zawartości rozpuszczonego tlenu.
18) Powłoka i gęstość prądu – Użycie powlekanych próbek pozwoli zademonstrować wpływ powłok na zanurzone lub zakopane konstrukcje i poda wielkość efektu, wyjaśniając, że synergia między ochroną katodową a powłoką chronionych powierzchni zmniejszy gęstość prądu ze wszystkimi istotnymi zaletami.
Lista materiałów:
- Ławka na kółkach z konsolą elektryczną do podłączenia do sieci zasilającej Vac i
- zamykane półki do przechowywania wymienionych poniżej materiałów. Wyposażona w wodoodporną powierzchnię górną.
- okulary ochronne i odblaski.
- Woltomierz cyfrowy.
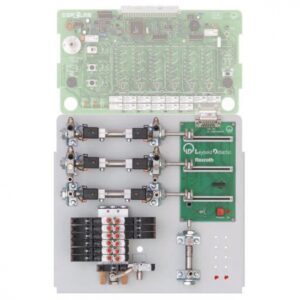
- Interfejs PC do pomiaru i przechowywania danych.
- Cyfrowy woltomierz na konsoli.
- cyfrowy amperomierz na konsoli.
- Cela referencyjna Cu/CuSO4.
- Ogniwo referencyjne Ag/AgCl.
- ogniwo referencyjne Zn.
- elektroda miedziana
- elektroda ze stali węglowej
- przezroczysta miska do budowy elektrolitycznej kąpieli testowej
- Prosty obwód z rezystorem przesuwnym i lampą odpowiedni do wstawienia do obwodu elektrycznego
- ogniwa elektrolitycznego.
- Elektroda cynkowa
- elektroda magnezowa
- Elektroda aluminiowa
- Podajnik prądu stałego (każdy wyposażony w urządzenia o stałym napięciu, stałym prądzie i stałym potencjale)
- Anody Ti-Pt
- Anoda rurowa MMO
- Pręty Cu
- Pręty Fe
- Pręty Fe-Ni
- Płynne ogniwo oporowe
- Wodoodporny rezystor z urządzeniem termostatycznym
- Pompa powietrza z odpowiednim rozpylaczem
- elektroda ze stali węglowej (całkowicie pokryta związkiem epoksydowym)
- elektroda ze stali węglowej (częściowo pokryta związkiem epoksydowym)
- różne odczynniki w plastikowych puszkach z kartą techniczną zgodnie z wymaganiami CE.
- Zestaw zapasowych bezpieczników.
- Zestaw akcesoriów i przewodów połączeniowych
- papierowe kopie i płyta CD podręcznika do szkolenia nauczyciela w celu przeprowadzenia eksperymentów.